Infrared spectroscopy (IR spectroscopy) refers to the branch of spectroscopy that focuses on the infrared portion of the electromagnetic spectrum, which consists of light characterized by longer wavelengths and lower frequencies than those of visible light.
This field encompasses a variety of techniques, primarily centered around absorption spectroscopy.
Capabilities of Infrared Analysis
- Identification and quantification of organic samples in solid, liquid, or gaseous states.
- Analysis encompasses powders, solids, gels, emulsions, pastes, pure liquids, and solutions, as well as polymers and both pure and mixed gases.
- Infrared technology is employed for research purposes, method development, quality control, and quality assurance applications.
- Sample sizes vary from individual fibers measuring merely 20 microns in length to extensive atmospheric pollution studies covering large areas.
Applications of Infrared Analysis
- Pharmaceutical research
- Forensic investigations
- Polymer analysis
- Lubricant formulation and fuel additives
- Foods research
- Quality assurance and control
- Environmental and water quality analysis methods
- Biochemical and biomedical research
- Coatings and surfactants
- The IR region is commonly divided into three smaller areas: near IR, mid IR, and far IR.
Care and Handling of IR Plates
The infrared plates utilized in organic chemistry laboratories are constructed from polished sodium chloride. Sodium chloride is selected due to its transparency to infrared radiation. These plates, referred to as “salt plates,” are relatively costly as each one is sliced from a single large crystal; they are quite delicate and susceptible to moisture, including the moisture present on your fingers.
What occurs when salt is added to water? It dissolves. What occurs when you press your sweaty fingers against a salt plate? It dissolves the salt, resulting in a fingerprint on the plate.
Humidity in the atmosphere leads to the cloudiness of salt plates; moisture on your fingers results in fingerprints. We keep them in desiccators to avoid cloudiness. Salt plates are extremely delicate and may chip or break if dropped. They are susceptible to scratches from metal spatulas and Pasteur pipets. The image below displays five plates, one in excellent condition and four that exhibit minor damage. When preparing to conduct a spectrum, it is advisable to select a good plate (and maintain its condition!). All the plates depicted in the image are likely usable, particularly for applications in organic teaching laboratories.
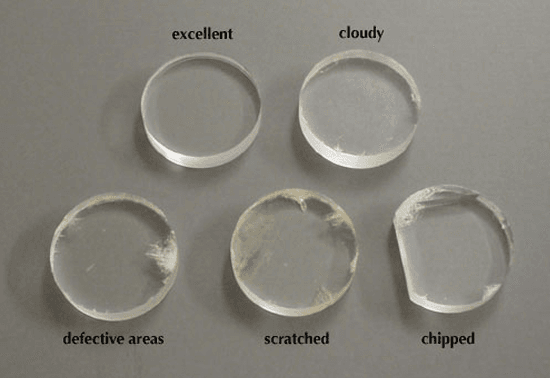
The harm inflicted on a plate by fingerprints, pits, and cloudiness results in the IR spectrum obtained from the plate exhibiting broad bands. This occurs because IR spectra are most effective when measured as thin, uniform films of compounds. The presence of pits and fingerprints creates a thick and uneven film on the plate, which leads to the scattering of IR radiation and produces bands that are excessively intense; additionally, cloudiness causes further scattering of IR radiation, obstructing its passage through the sample, which results in broad bands and a spectrum that does not achieve 100% transmission.
Can plates still be utilized despite being damaged? Generally, the response is “yes”. If the final plate intended for use appears cloudy or pitted, attempt to run a spectrum to determine if the results are satisfactory. A plate with a significant chip can still be employed, provided it is sufficiently large to rest on the holder and within the irradiation path. IR plates are capable of being resurfaced, so do not discard a plate simply because it appears unsightly.
Thin-film IR Sampling Techniques
Two distinct methods are employed to create thin films on an IR plate. If the substance under investigation is a liquid, the thin-liquid film IR sampling technique should be utilized. Conversely, if the substance is a solid, the thin-solid film IR sampling technique is required. It is important to note that a solution of a solid compound in a solvent does not qualify as a liquid; if the compound of interest remains solid at room temperature, even when dissolved in a solvent such as methylene chloride, it is still classified as a solid and must be processed according to the thin-solid film procedure.
After the sample has been prepared, the spectrum can be collected using one of the IR instruments.
Other Sampling Techniques
Solid samples can be prepared for infrared (IR) examination using various methods:
- A mull is created by grinding the sample with mineral oil and then sandwiching the resulting paste between sodium chloride plates.
- The solid may also be dissolved in a solvent and placed in a specialized cell, known as a solution cell, which is constructed from sodium chloride. This process yields what is referred to as a solution spectrum.
- A KBr pellet is formed by grinding the solid sample with solid potassium bromide (KBr) and applying significant pressure to the dry mixture. KBr is selected for this purpose due to its transparency to infrared radiation. When the pellet is prepared correctly, it is possible to see through it, much like looking through a pane of glass.
